Electrochemical Impedance Spectroscopy (EIS)
Electrochemical Impedance Spectroscopy (EIS) is an electrochemical technique to measure the impedance of a system in dependence of the AC potentials frequency.
Electrochemical Impedance Spectroscopy: complex and popular
Electrochemical Impedance Spectroscopy (EIS) is one of the most complex techniques in electrochemical research. On this page the basics of EIS are explained, i.e. the excitation and the signal as well as the recorded values. The visualization and analysis of the EIS data is explained in the following chapters.
Electrochemical Impedance Spectroscopy (EIS) gained a lot of attention in the last 10 years. It is for multiple reasons quite popular. One reason is that EIS allows separating the influences of different components that means the contribution of the electron transfer resistance, double layer capacity, etc.

Surface sensitive
Another one is that electrochemical impedance spectroscopy is very surface sensitive, which makes many changes visible that other techniques don’t see, for example changes in polymer layers due to swelling, surface changes due to protein adsorption or penetration of corrosion protection layers.
As a result electrochemical impedance spectroscopy is interesting for analytical electrochemistry, because molecules can be detected without a redox active marker.
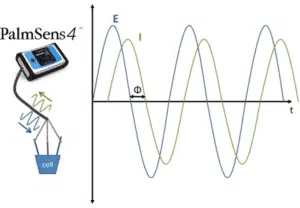
While resistance is the ratio of voltage or potential and current for a DC (direct current) system, the impedance is the ratio of voltage or potential and current for AC (alternating current) systems.
The wave nature makes it necessary to define the impedance with two parameters. One is the total impedance Z and the other one is the phase shift Φ.
Periodic waves of current and voltage
If you consider the two periodic waves of current and voltage, the waves have the same frequency, because one wave causes the other. There is a constant time shift between the two waves, which is called the phase shift Φ. Its unit is degrees (°), because usually waves are considered to be vectors in a polar coordinate system or a sine function (see Figure 1.1).
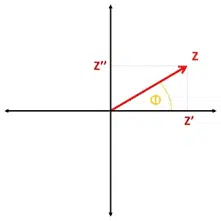
The total impedance is the ratio of the potential’s amplitude and the current’s amplitude. The resulting impedance is a complex number. This number can be expressed in the complex plane in polar coordinates by using Z as the length of the vector and Φ as the angle.
With the common knowledge about calculations for complex numbers the impedance can also be expressed as the real part of the impedance Z’, which is the resistance, and imaginary part Z’’ (see Figure 1.2).
Bode plot and Nyquist plot
The two notations are the origin of the two most popular plots for impedance spectra: the Bode plot and Nyquist plot. More information is given here: Bode and Nyquist Plot.
A potentiostat measures the impedance by applying a potential wave to the working electrode and records the resulting current wave. From these two waves the potentiostat calculates Z, Φ, Z’ and Z’’. The spectrum is made by measuring these parameters for potential waves with different frequencies.
Frequencies
A fixed number per decade is usually chosen, because most plots have a logarithmic axis. This means for example 10 frequencies between 10 000 Hz and 1000 Hz, 10 between 1000 Hz and 100 Hz etc. are chosen, these frequencies are usually equidistant on a logarithmic scale.
PSTrace allows you to choose, if you want to define the total number of points through the whole spectrum or the number of points per decade, whatever option you choose, you can always see a list of the chosen frequencies.
- Electrochemical Impedance Spectroscopy
- Electrochemical Impedance Spectroscopy (EIS) is an electrochemical techniques to measure the impedance of a system in dependence of the AC potentials frequency.
See PalmSens4 with EIS up to 1 MHz Compare instruments with EIS
See also:
Frequently Asked Questions
What is Electrochemical Impedance Spectroscopy (EIS)?
Electrochemical Impedance Spectroscopy (EIS) is an electrochemical technique to measure the impedance of a system in dependence of the AC potentials frequency. It is for multiple reasons quite popular.
One reason is that EIS allows separating the influences of different components that means the contribution of the electron transfer resistance, double layer capacity, etc.
Another one is that electrochemical impedance spectroscopy is very surface sensitive, which makes many changes visible that other techniques don’t see, for example changes in polymer layers due to swelling, surface changes due to protein adsorption or penetration of corrosion protection layers.
As a result electrochemical impedance spectroscopy is interesting for analytical electrochemistry, because molecules can be detected without a redox active marker.
What is Electrochemical Impedance Spectroscopy (EIS) used for?
Electrochemical Impedance Spectroscopy is used to demonstrate the presence of a particular substance in a (usually a) liquid and/or to measure its quantity using electrical waves.
A potentiostat sends these waves to the liquid to be measured and from the reaction that comes back an electrochemical researcher can read the information he needs for this purpose.
One can think of testing/measuring HIV in blood or mercury in groundwater. The advantage of electrochemical impedance spectroscopy is that the testing does not affect the fluid, the testing can be done outside the lab and the testing takes little time.
