Anodic Stripping Voltammetry
Anodic Stripping Voltammetry is a method to demonstrate the presence of multiple metals in water. This method is usually applied to investigate the water quality of sewage, surface or drinking water.
Anodic stripping voltammetry
For Anodic Stripping Voltammetry several steps need to be performed during which low, medium and high potentials are applied to an electrode in a certain volume of water.
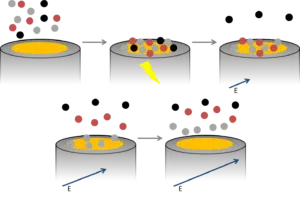
First a high potential is applied to clean the electrode. Then the sample (water) is stirred for a certain period (usually between 60 and 300 seconds) at a low potential. With this low potential, all dissolved metal ions in the water are reduced and remain on the electrode as a metal film.
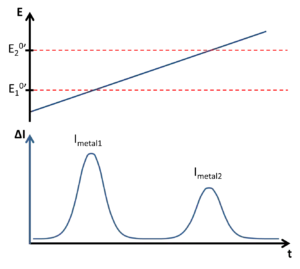
After this time the stirring is stopped and the electrode is covered with the different metal particles. Their amounts are measured with a potentiostat, the potentiostat linearly increases the potential and at each specific redox potential of a specific metal, this metal oxidizes on the electrode surface. Each time one of the metals is oxidized an electrical current is measured by the potentiostat (Figure 2).
When the values found are displayed in a graph (Figure 1), this graph will show various peaks (see below) over time where the changing time also represents the potential change from E1 to E2. These peaks each stand for amount of metals at the redox potential associated with that certain kind of metal.
Advantages of Anodic Stripping Voltammetry
Anodic Stripping Voltammetry allows researchers to detect multiple types of dissolved metal in one experiment. Researching water quality using Anodic Stripping Voltammetry is also fast and accurate. An Anodic Stripping Voltammetry test is very easy to perform on site by taking a sample of the water and testing it with the potentiostat linked to a laptop or tablet.
The metal film formed during the reduction step concentrates the metal particles at the electrode, so the detection of very low concentrations (ppb range) of metal ions is possible.
Applications of Anodic Stripping Voltammetry
Anodic Stripping Voltammetry is widely used for testing drinking water quality, surface water and sewage or waste water – water that leaves the treatment plant before being discharged into surface water.
There are also known applications in which the water quality in the sewage pipes is continuously measured, using a clever underground test rig. When a pipe starts to corrode, this is immediately noticed by means of the increased concentration of metals in the water. Upon detection it is then possible to intervene in good time.
What do you need for Anodic Stripping Voltammetry?
To perform a water quality test you only need a potentiostat, such as the EmStat4X with a sensor sensitive to metals. When you connect the potentiostat to a laptop, tablet or smartphone and use the included user-friendly software, you can immediately measure and analyze results.
- Anodic Stripping Voltammetry
- A method to demonstrate the presence of multiple metals in water. This method is usually applied to investigate the water quality of sewage, surface or drinking water.
How to perform Anodic Stripping Voltammetry?
Let our electrochemist Lutz Stratmann explain how to perform stripping techniques in PSTrace. PSTrace is a powerful software packages and comes included with all our potentiostats.
