Ohmic drop
Positioning of the reference electrode is often not important, if you have a well-conducting solution. If you have a solution with low conductivity, you might experience Ohmic drop. The Ohmic drop is the amount of potential that is lost on the way from the reference electrode to the working electrodes.
The Ohmic drop is a result of the Ohmic resistance between the reference electrode and working electrode. The resistance depends on the distance between the electrodes, the conductivity of the solution and the frits used. The frits are fixed and can’t be influenced by the user.
The conductivity of the solution is often dictated by the experiments. In a solution of 100 mM KCl is the conductivity sufficiently high, that for most purposes the Ohmic drop is negligible. Corrosion studies sometimes require that the solution is chosen according to the real environment the sample will be exposed to, which could mean a low conducting solution. Here Ohmic Drop could be important.
Articles

Purpose of a Sense lead
Sense leads also known as working sense leads are common for many potentiostat brands. PalmSens uses the Sense lead on all models that can supply 100 mA or more. This raises the qu...
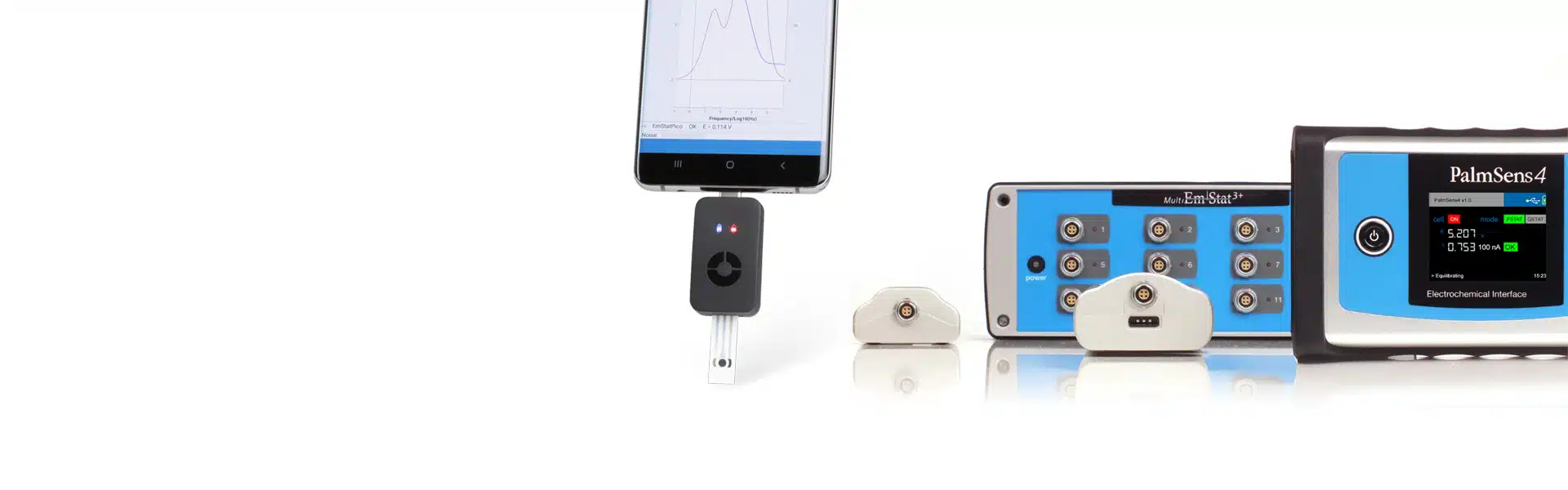
Portable potentiostat
In some cases you want to carry out your measurements in the field, in several places and have results available immediately. That is why PalmSens has developed a portable battery-powered potentiostat and a USB-powered potentiostat that you can connect directly to your phone.

Potentiostat
A potentiostat is a device that can demonstrate the presence of a certain substance by measuring electrical current. Learn how it works
