Copper and Nickel Deposition 2/3 – Corrosion research and corrosion protection
This chapter is part of the series ‘Copper and Nickel deposition ’. In this chapter, we discuss in detail what corrosion entails, and how we can protect materials against it.
Buildings, bridges and monuments
Corrosion is the destruction of materials by chemical reactions with the environment. It is a big cost factor. Replacement of corroded materials in buildings, bridges, or monuments and painting or galvanization of building elements is a huge investment into maintenance.
The study “Corrosion Costs and Preventive Strategies in the United States” showed that in 1998 corrosion cost the U.S. $276 billion. This is ca. 3.2 % of the US gross domestic product [1]. Painting the Eiffel tower costs ca. €4 million every 7 years [2]. Without a proper and closed layer of paint this monument would become unstable and break down.
As a consequence, one of the most important fields in electrochemical research focuses on corrosion. A lot of companies fund academic research for investigating the mechanism of corrosive processes and for creating new methods for corrosion protection. Companies check paints and lubricants for their corrosion protection capabilities and develop new methods for creating coatings or creating non-corrosive environments for machinery, for example flooding the entire gears with oil.
From a chemical point of view, protection by ordinary paints is easy to understand. The paint is usually a mixture of a polymer, color pigments, and various additives for adjusting the physical properties of the paint. For example these additives create smooth or rough surfaces or grant UV-light protection. The paint is usually dispersed or dissolved in some solvent (water or an organic solvent). The solvent evaporates after the paint has been applied and a polymer with the additives and pigments stays on the painted surface.
The result is a physical barrier between the surface and its environment. This barrier protects the surface from corrosion. A crack in the layer of paint will grant access to the surface and corrosion may occur.
Opportunities for electrochemistry in corrosion research
Here already electrochemistry can help improve the protection layer. Electrodeposition paints are paints that are made insoluble by an electrochemical reaction. A conducting work piece is dipped into the paint bath and a potential is applied. The created reaction close to the work piece’s surface will make the paint insoluble and thus a layer of paint precipitates on the metal surface. All conducting parts will be covered with the paint, even usually hard to reach places.
If the paint forms an insulating layer, the reaction will stop itself by blocking the conducting surface completely; only the needed amount of paint will be used. These paints have different mechanisms.
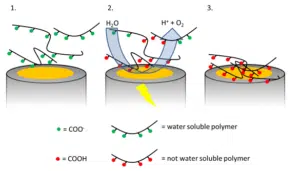
The most common one is neutralizing charges. The polymers in the paints have some charged functional groups. These groups make them water soluble. These groups can be deprotonated acids or protonated bases. By way of illustration we assume a polymer with deprotonated carboxylic groups (COO–) is used. Due to its negative charge it will migrate towards the positive charged electrode, the anode. The potential at the anode is high enough for water to split as follows:
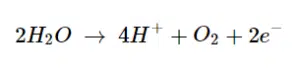
The high concentration of H+, which implies a low pH value, close to the anode leads to a protonation of the COO– groups.
As a result the polymer’s COOH groups have no charge and the polymer is not soluble in water anymore. The polymer precipitates on the anode (see Figure 1.1). After completion of the deposition excess paint is rinsed away and the paint layer is cured. This means that the paint is heated up, so it can flow and form a uniform surface.
Corrosion protection becomes easier if the metal itself forms a protective layer. Aluminum oxide layers on aluminum are impenetrable for oxygen and thus protect the aluminum layer beneath. This has the advantage that damaged parts of the coating will be replaced by the aluminum itself. The thickness of the layer can be increased electrochemically, leading to a better protection.
Corrosion protection: active coatings
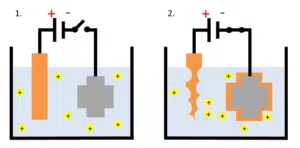
Up to now, the mentioned coatings were passive coatings. Active coatings can be created by covering metals with metals. This is usually done by dipping the metal to protect in a solution with a salt of the coating metal. A potential is applied between the metal that needs protection and an electrode made from the coating metal.
A negative potential is applied to the core metal and all positive metal ions (cations) will migrate to the core metal that acts as cathode, the electrode where reduction takes place, and the ions are reduced to elemental metal at the cathode. The other electrode is oxidizing its own metal and releasing it as ions into the solution. This electrode is called anode, the electrode where oxidation happens (see Figure 1.2).
Coating with noble metals
Due to knowledge of the electrochemical series one can predict if a certain metal is nobler than another one and thus predict its behavior in contact with it. Noble metals are useful to create a coating that is inert to corrosion, but if the coating gets damaged and the less noble metal is exposed, a local element is created. The noble coating metal will take electrons from the less noble core metal as a result the speed of corrosion is increased instead of slowed down. An example of this would be chromium coated steel.
Coating with a less noble metal that passivates itself is usually a better idea. In case the coating is damaged and the core metal is exposed the coating will act as a sacrificial anode. The noble core metal will drain electrons from the less noble coating and thus the core will reduce itself, while the coating is oxidized. An example of this is zinc coated steel.
There are more ways to protect a product from corrosion, but within the framework of this experiment the knowledge of these most important methods will suffice.
Literature
[1] Gerhardus H. Koch, Michiel P.H.Brongers, Neil G. Thompson, Y. Paul Virmani and Joe H. Payer. Corrosion Costs and Preventive Strategies in the United States – report by CC Technologies Laboratories, Inc. to Federal Highway Administration (FHWA), September 2001.
[2] http://www.toureiffel.paris/en/everything-about-the-tower/themed-files/97.html.
