Detection of Glucose with a Self-Made Biosensor 5/5 – Glucose and Glucose Oxidase
This chapter is part of the series ‘Detection of glucose with a self-made biosensor based on glucose oxidase’. This chapter is the final theoretical part, which elaborates on the experiment with the enzyme Glucose Oxidase (GOx).
Glucose Oxidase
Glucose Oxidase (GOx) is one of the best-researched and popular enzymes. This has two main reasons. The first reason is the commercial success of the glucose sensors for diabetes patients. This leads to potential funding for projects with the objective to improve glucose sensing. And using GOx in your experiment will already imply an application. The other reason is GOx’s high stability. Compared to many other enzymes GOx is not denaturized easily. It usually stays active during most immobilization techniques.
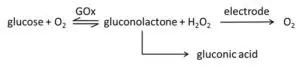
In this experiment the production of hydrogen peroxide as a stochastic byproduct by GOx will be exploited (Figure 4). The hydrogen peroxide diffuses towards the electrode and is oxidized at the electrode. After the GOx has oxidized the glucose to gluconolactone, the GOx transfers the electrons from the Glucose to oxygen reducing it to hydrogen peroxide. Gluconolactone is hydrolyzed in aqueous solutions to gluconic acid. There are even glucose sensors that exploit the induced pH change.