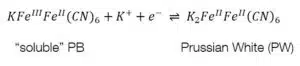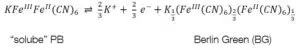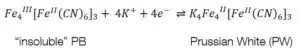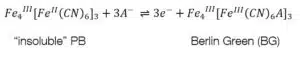Detection of Hydrogen Peroxide 3/5 – What is Prussian Blue?
This chapter is part of the series ‘Detection of hydrogen peroxide with Prussian Blue’. This chapter covers Prussian Blue and its characteristics.
Prussian Blue
Prussian Blue (PB) is a deep blue pigment that is hardly soluble in water, but little amounts that are dissolved or dispersed in watercolor the solution intensively blue. Small Prussian Blue particles dispersed in water have been used as the first modern synthetic color. It is also known as Berlin Blue or Turnbull’s Blue. The pigment is used for various paints (Paris Blue) or to make the blue color for blueprints.
Prussian Blue is also used for capturing certain heavy metals inside the human body, thus preventing metal poisoning. Prussian Blue can do this because it is a complex with cyanide ligands.
More specifically, a complex made from iron and cyanide ions. Cyanides are the salts of prussic acid, also known as hydrogen cyanide (HCN). This dangerous substance is known from criminal fiction or spy stories for blocking the respiratory mechanisms of the cells. In Prussian Blue cyanide is so tightly bound that it can be consumed in certain doses without any hazard.
The iron ions are present as Fe2+ and Fe3+ depending on the form of Prussian Blue. Prussian Blue is produced from iron chloride and hexacyano ferrate. The latter is a complex with an iron center surrounded by six cyanide ions [FeII(CN)6]4-. The cyanide ions form the corners of an octaeder. It is pale yellow if the iron is Fe2+, orange if solid, or bright yellow when in a solution the iron is Fe3+. Due to the negative charge of the cyanide ions (CN–) the complex is negatively charged.
To form one of the forms of Prussian Blue (PB) one of the counter ions must also be an iron ion. The most common form is the “soluble” PR KFeIIIFeII(CN)6. The “insoluble” form is Fe4III[FeII(CN)6]3. To avoid confusion, it needs to be clarified that “soluble” and “insoluble” are not referring to the solubility of PB, but to how easy they can be dispersed in water. The names of the forms were given by dye makers. Neither forms are soluble as it is usually used in chemistry. After the first electrochemical deposition of Prussian Blue in 1978 its electrochemical properties were fully investigated in less than 10 years.
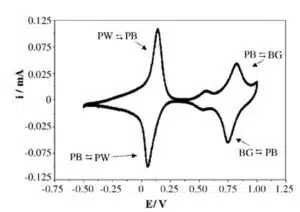
Prussian Blue can be reduced to Prussian White (see equations 4.1 and 4.2) or oxidized to Berlin Green (see equations 4.3 and 4.4). These changes can be observed in the CV of Prussian Blue (see Figure 4).
Literature
[1] F. Ricci, G. Palleschi, Biosens Biolectron 21 (2005), p. 389-407.