Corrosion potential
Corrosion can only occur if the electrons donated by one reaction equal the electrons accepted by another system. For example, the electrons donated by an iron surface must be equal to the electrons accepted by the surrounding oxygen.
The electrons transferred between these two systems define the corrosion current. Usually two electrochemical reaction (one reduction and one oxidation) have only one potential, where the current of both systems is equal. This potential is the corrosion potential Ecorr.
A simpler way to express the corrosion potential is: The corrosion potential is the potential where the corrosion current is flowing.
A corroding systems Open Circuit Potential (OCP) is usually the corrosion potential. This is why in the Corrosion Mode of PSTrace the OCP measurement is renamed to Ecorr measurement.
More detailed information about the corrosion potential and corrosion current is available in the knowledge database article.
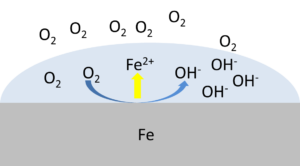
- Corrosion potential
- The corrosion potential is the potential where the corrosion current is flowing.
Articles
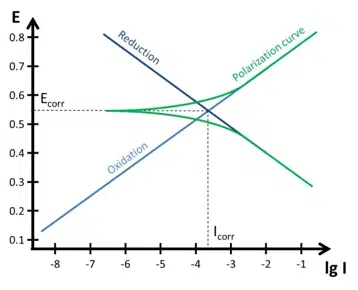
Corrosion Potential
In this chapter the Open Circuit Potential (OCP) and the Corrosion Potential are introduced. The principles of reference electrodes as well as the 0 V by convention potential of the standard hydrogen electrode (SHE) are explained.
