Faraday's law
Faraday’s law is next to the Nernst Equation the most important equation for electrochemistry. It describes the connection between the charge Q passing through a cell and the amount of substance n converted at the electrodes:
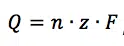
F is the Faraday constant and z the number of electrons needed per conversion.
Articles
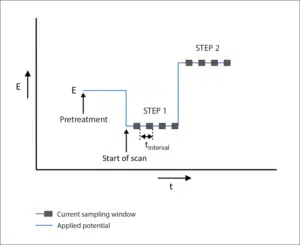
Coulometric Detection
Coulometric Detection is an electrochemical technique during which a potential is set. This technique is usually called Chronocoulometry at PalmSens. The current is recorded and shown in a graph versus the time.
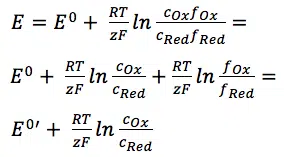
The equations behind the potentiostat
This article explains the working of a potentiostat more in depth, using Faraday’s law and the Nernst equation. For the basics of the potentiostat, please read the potentiost...

Chronocoulometry (CC)
Chronocoulometry is an electrochemical technique during which a potential is set. The current is recorded and shown in a graph versus the time.
