Chronocoulometry (CC)
Chronocoulometry is an electrochemical technique during which a potential is set. The current is recorded and shown in a graph versus the time.
Description
Chronoamperometry (CA) and chronocoulometry (CC) have the same potential waveform but are used for different purposes. In CA, the current is monitored as a function of time, whereas in CC, the charge is monitored as a function of time. This means that in essence, the potentiostat performs the same experiment as in CA, recording the current as a function of time.
However, the current is displayed in the software as a charge versus time. The charge is calculated by integrating the current. While the measured current in the CA shows a linear correlation with the reaction rate, the charge measured in CC shows a linear correlation with the number of converted reactants. This is described in Faraday’s law.
Use
Chronocoulometry (CC) is useful during deposition or electrochemical synthesis. For the characterization of batteries, CC is also helpful. In analytical chemistry, CC is used to determine the adsorbed number of active species in a solution of free diffusing active species. Multiplying the Cottrell equation with t delivers that the charge Q of the free diffusing species’ reaction is proportional to t½. The total charge also includes the contribution of the charge stored in the electrochemical double layer and the charge due to reactions of adsorbed species.
These two effects are a lot faster than the reaction of free diffusing species. Plotting Q versus t½ (Anson plot) delivers (ideally) a jump in charge followed by a linear increase. When the linear part is extrapolated, the intersection with the charge axis delivers the contribution of the double layer and adsorbed species. A previous blank measurement allows the determination of the double layer contribution and thus the calculation of the adsorbed species contribution.
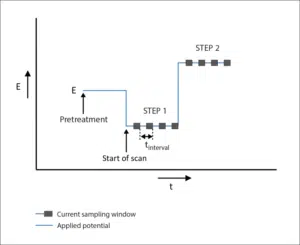
To make sure the charge step, in the beginning, is recorded this measurement is performed in two steps. The first step at a reaction-free potential followed by the step where the reaction is initiated, just like the classic Cottrell experiment.
Signal applied during Chronocoulometry.
Application
Chronocoulometry can be applied with the following instruments from PalmSens:
Frequently Asked Questions
What is the use of Chronocoulometry?
Chronocoulometry (CC) is useful during deposition or electrochemical synthesis. For the characterization of batteries, CC is also helpful. In analytical chemistry, CC is used to determine the adsorbed number of active species in a solution of free diffusing active species.
What is the difference between Chronoamperometry and Chronocoulometry?
Chronoamperometry (CA) and chronocoulometry (CC) have the same potential waveform but are used for different purposes. In CA, the current is monitored as a function of time, whereas in CC, the charge is monitored as a function of time. This means that in essence, the potentiostat performs the same experiment as in CA, recording the current as a function of time.