Nyquist plot
During an Electrochemical Impedance Spectroscopy (EIS) a lot of data is collected and this data needs to be presented. A picture says more than thousand words and so a graphical representation, such as a Nyquist plot, is the best way to show an EIS.
The most popular plots for EIS data are the Bode plot and the Nyquist plot. This article focuses on the Nyquist plot. It is named after Harry Nyquist, a Swedish-American electrical engineer, and developed in 1932 for electronics purposes. Nowadays it is the most popular plot for EIS data among electrochemists.
Advantages of the Nyquist Plot
The plot shows the negative imaginary part of the impedance versus the real part. Compared to the Bode plot it has two disadvantages:
- It is less intuitive to understand.
- The frequency information is missing.
However, the Nyquist plot shows a characteristic shape depending on which components contribute to the impedance. The Nyquist plot shows very clearly even small changes in the studied surface.
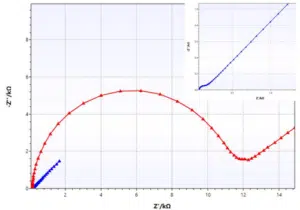
With a bit of experience one can read some parameters directly from the Nyquist plot like the solution resistance or charge transfer resistance, so the resistance of the surface to perform a certain reaction.
For a more precisely analysis Equivalent Circuit Fitting is performed. Here the influences on the impedance are represented by electronic components (an Equivalent Circuit) and the values of each influence is calculated by fitting a simulation of the data to the real data.
Applications
Some studies just follow how the charge transfer resistance changes others observe the change of the complete system.
An example for the first one is label-free detection of DNA. With EIS the hybridization of complementary surface bound DNA strands can be observed in a Nyquist plot and thus bacteria, viruses, etc. can be detected. For other electrochemical techniques an active species needs to be introduced, which changes the DNA and costs time.
Corrosion studies with a Nyquist Plot
A very different field of application is the study of corrosion. EIS is especially popular to research coatings and paints.
A perfect coating will deliver a vertical line in a Nyquist plot, while a coating penetrated by water shows a semi-circle and corrosion under the coating has another shape. This way the status of coated metal can be evaluated and the water uptake of the coating can be determined.
These are just two examples and there are many more applications for example more label-free detection, battery and fuel cell research.
Articles
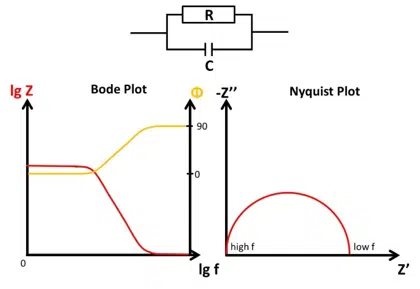
Bode and Nyquist Plot
In this chapter the two main ways of visualizing Electrochemical Impedance Spectra (EIS), the Nyquist and Bode plot, are presented and it is explained how different EIS of easy electronic circuits will be plotted in the Bode and Nyquist plot. This demonstrates the advantages and disadvantages of the two plots as well as serving as a foundation to understand the analysis of EIS by utilizing equivalent circuits.
