Cyclic Voltammetry 4/4- Investigation of Catalytic Processes
This chapter is part of the series ‘Cyclic Voltammetry – the Most Used Technique’. In this final chapter we delve into catalytic processes, whilst focusing on biosensors and cyclic voltammetry.
Catalysts
In industrial chemistry, catalysis is very important. Investment in good catalysts, compared to the costs of the rest of the process, is rather small and the raise in process efficiency is usually tremendous. As a result the funding by industrial partners for researching new catalysts or improving older catalysts is usually quite generous.
Catalysts are substances that lower the activation energy of a reaction without getting consumed during the reaction. Electrochemical catalysts have different applications. One important application is the fuel cell. Hydrogen is oxidized and oxygen is reduced, but these two reactions happen at different places. The electrons will travel through a cable from the hydrogen side to the oxygen side, so the fuel cell acts as a power source, and the ions will travel through an electrolyte or a membrane to close the circuit. Catalysts that facilitate the hydrogen or oxygen reaction improve the energy yield and make a commercialized use for fuel cells interesting.
Another field is sensor applications. A catalyst enables reactions to happen at lower potentials and thus the danger of side reactions is smaller. An example is Prussian blue modified electrodes. Usually, the reduction of hydrogen peroxide requires around ‑500 mV vs Ag/AgCl and in this range oxygen reduction occurs. The oxygen present in the solution will influence a hydrogen peroxide reduction, but a Prussian blue covered electrode reduces hydrogen peroxide already at 0 mV vs Ag/AgCl. This way, the detection of hydrogen peroxide has fewer side reactions.
Enzymatic biosensors
Catalysts are selective for certain reactions and the ideal catalyst has a high selectivity, that improves one reaction only. Enzymes are biological catalysts made out of proteins. They are usually highly selective and effective. Due to this, a lot of research is performed on biosensors to exploit these properties. Biosensors in general are sensors using a biological recognition element, for example enzymes, DNA, antibodies, etc., which reaction can be converted into an electronic signal. But for now, we want to focus on an enzymatic biosensor and how cyclic voltammetry contributes to its characterization.
Using enzymes for electrochemical measurements has a disadvantage. Most enzymes are large proteins and only a small part contains the electrochemical active part where electrons are “stored”. To measure an oxidation or reduction that is catalyzed by an enzyme electrochemically, the electrode will have to communicate with this active region called the active center. There are enzymes with active centers that allow a direct electron transfer from the active center to the electrode, but most enzymes do not allow this to happen. The protein shell of the active center insulates the active center.
A direct electron transfer was reported for example for cytochrome c; horseradish peroxidase; catalase; glucose oxidase; and xanthine oxidase. To achieve direct electron transfer, a special modification of the surface is often needed to create the right orientation of the enzyme and get the active center close enough to the electrode.
As an alternative, an indirect electron transfer via an electrochemical mediator can be applied. The mediator is a reversible redox couple that travels back and forth between the active center and the electrode (see Figure 3.1). The mediator has to be able to travel to the active center of the enzyme and diffuse to the electrode, and its potential has to be higher or lower than the potential of the active center to accept or donate an electron. In addition, the mediator will compete with the natural partner of the enzyme, which is often oxygen.
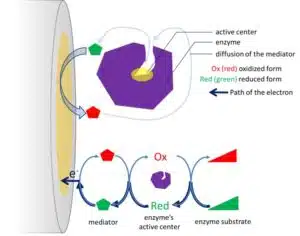
Catalysis in biosensors
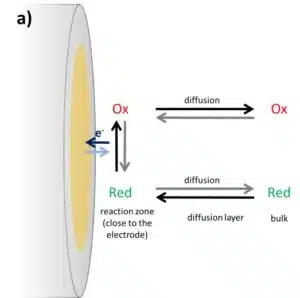
If a biosensor is developed, a suitable mediator must be found for the enzyme. Cyclic voltammetry can help to find a suitable mediator. A catalyzed process gives a very different CV than an uncatalyzed one. The concentrations of the different species in solution during an electrochemical experiment depend on the distance to the electrode surface, as seen in the chapter about the Cottrell experiment.
Directly in front of the electrode, the ratio between Ox and Red is determined by the Nernst equation (see also this chapter). Followed by the diffusion layer, where the diffusion towards the electrode determines the concentrations, and further away from the electrode, the starting concentration is still present in the bulk. The amount of active species consumed by the electrode depends on the potential, the concentration of already converted species, and the diffusion from the bulk towards the electrode. This is shown in Figure 3.2a. The black and dark blue arrows are the dominant paths for the oxidation at the electrode.
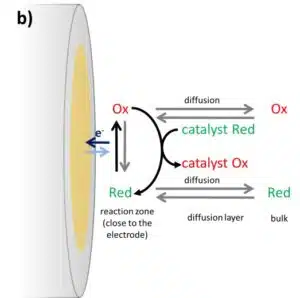
Usually, the species in front of the electrode is consumed during the potential sweep and its depletion will cause the current to drop again. The scan rate is at the vertex potential inverted and the converted species is consumed during the back reaction. If a catalyst is added, for example an enzyme, and the catalyst will recover the converted species’ original state the concentrations will be influenced in a different way. An example of an oxidation at the electrode is shown in Figure 3.2b.
Assuming that the catalyst and the species, which will be consumed by the enzyme to reduce the catalyst again, are present in high enough concentrations the oxidized form of the mediator Ox will be reduced by the catalyst immediately. This has two major influences on a CV. The first one is the absence of a reduction peak in the CV, because all the oxidized species is already reduced by the catalyst.
The second change in the CV is the increase in anodic current without a decrease in current due to depletion of the reduced form (Cottrell behavior). The oxidized form of the mediator is always immediately reduced by the catalyst, so in front of the electrode one always finds the reduced form. This does not only prevent depletion, it also creates a virtually higher concentration of the reduced form, leading to a higher current than the uncatalyzed CV. In ideal circumstances, this leads to a very big difference between the catalyzed and uncatalyzed CV (see Figure 3.3). The ratio of the peak current Ip,a and the catalyzed peak current Icat gives information about the kinetics between the mediator and the catalyst, but within the framework of this experiment the detailed calculation of the kinetics will not be covered.
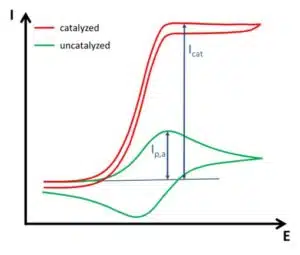
In real-life systems, the catalytic effect is often weaker and an influence of depletion will still be visible. The presence of the natural pendant for the mediator is usually a major interference. For many enzymes this is oxygen. Oxygen can be removed from the measuring solution by a constant stream of nitrogen, argon, or other inert gases through the solution. This usually improves the detection of the catalytic activity.