Potentiostat: a simple explanation
An electronic device that measures and controls the potential (or voltage) difference between two electrodes is called a potentiostat. These electrodes can be very small like micro-electrodes in a conductive solution, but also a coated metal coupon in an acidic environment. A three-electrode setup is also possible. A potentiostat can be used in the fields of electrochemistry and biochemistry, but also sensor development and battery research.
Video: What is a potentiostat, why use it and how to connect?
What is a potentiostat?
The word potentiostat comes from potential. In electronics and electrochemistry a potential is stored energy or the “potential to do work”.
Applying a potential with a potentiostat, for instance a PalmSens4, to a conducting surface in a conducting solution can induce a particular electrode current which is measured by the potentiostat while keeping the applied potential constant.
The advantage of a potentiostat is that it has complete control over the applied potential, unlike a normal power source.

What is a potentiostat used for?
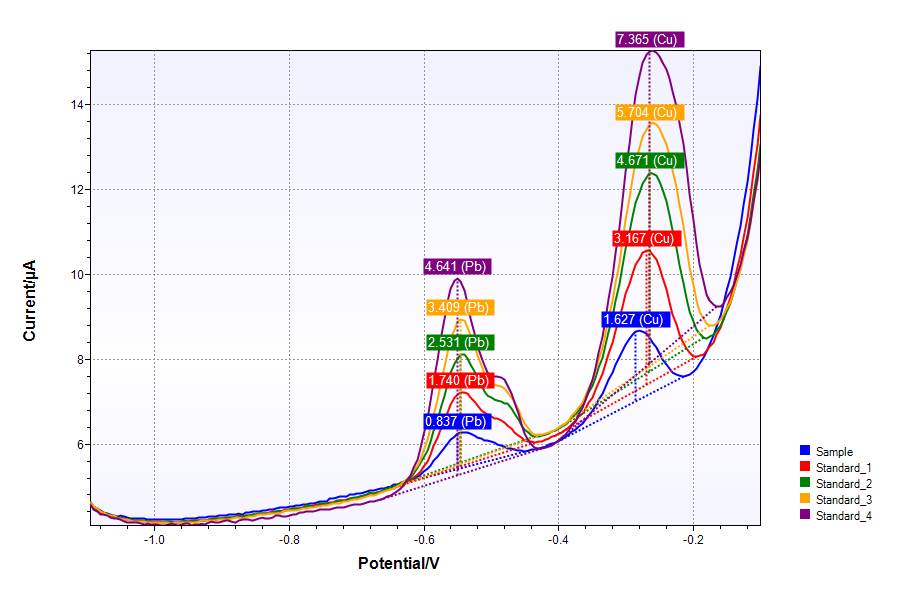
A potentiostat is used mainly in electrochemistry. For example, electrochemical researchers want to show how much lead or other heavy metals are present in drinking water (Figure 1), how much iron there is in blood or investigate how rainwater affects the surface of a certain metal (e.g. resulting in corrosion).
How does a potentiostat work?
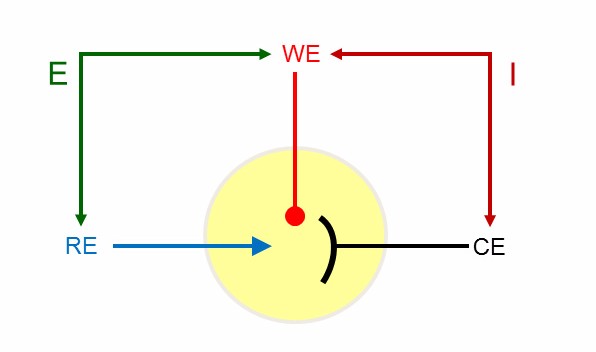
A potentiostat applies potential to a certain surface, an electrode. The amount of electrons on the surface is thereby reduced or increased. This causes the liquid to be triggered to deliver or consume electrodes to compensate for this. The exchange of electrons per time, the electrode’s current, can be measured by the potentiostat. This is usually done in a three-electrode setup (Figure 2).
An electrode made to deliver current of a certain substance is present is called a sensor. This sensor has a certain sensitivity to the substance that you want to measure. For this reason, there are many different sensors that react in different ways to certain chemical substances.
Sometimes a sensor can be used multiple times, but often they are disposable, so for a one-time use, and the sensor is permanently changed by the reactions happening at the electrodes.
Different types
There are several types of potentiostats used by electrochemical researchers.
- Most researchers in a university or laboratory use a potentiostat to detect all sorts of possible substances in fluid (for example, the PalmSens4)
- But there are also potentiostats that are only used to detect one particular substance. For this purpose PalmSens makes potentiostat modules, so-called OEM potentiostats.
- PalmSens also provides tailored potentiostats for specific applications like the EmStat Go.

Software
Without associated software, there is little to do with your portable potentiostat. PalmSens portable potentiostats are sold including intuitive software, which makes it easy to get started and it can be installed on multiple computers and laptops.
PalmSens also provides an app for Android devices, you can download this free of charge in the Google Play Store. You can also use all of the currently available spreadsheet software that is useful for creating charts using the Export Data functionality in PSTrace.
Potentiostat prices
Portable potentiostats usually come at a certain price. However, PalmSens managed to develop a series of portable potentiostats that are highly affordable, definitely with all the given specifications. E-mail us for a quotation or call us to discuss the possibilities for your research with us.
Request a quotationPotentiostat techniques
Overview of electrochemical techniques that can be performed using a potentiostat.
|
Voltammetric techniques |
Pulsed techniques |
Galvanostatic techniques |
Amperometric techniques |
Potentiostatic/Galvanostatic Impedance spectroscopy (EIS/GEIS) |
Other techniques |
|
|
|||||
Definition of a potentiostat
- Potentiostat
- An electronic device that measures and controls the potential (or voltage) difference between two electrodes is called a potentiostat. The advantage of a potentiostat is that it has complete control over the applied potential, unlike other power sources, while measuring the response current.
Frequently Asked Questions about a potentiostat
What is a potentiostat?
The word potentiostat comes from potential. In electronics and electrochemistry a potential is stored energy or the “potential to do work”.
Applying a potential with a potentiostat, for instance a PalmSens4, to a conducting surface in a conducting solution can induce a particular electrode current which is measured by the potentiostat while keeping the applied potential constant.
The advantage of a potentiostat is that it has complete control over the applied potential, unlike a normal power source.
Which potentiostat do I need?
An electrochemist typically spends many months together with a potentiostat to get research done. The potentiostat’s performance should not limit the results and the software should be user friendly and easy to use. Here are six factors to consider before buying a potentiostat. Read more
What is a potentiostat used for?
A potentiostat is used mainly in electrochemistry. For example, electrochemical researchers want to show how much lead or other heavy metals are present in drinking water (Figure 1), how much iron there is in blood or investigate how rainwater affects the surface of a certain metal (e.g. resulting in corrosion).


How does a potentiostat work?
A potentiostat applies potential to a certain surface, an electrode. The amount of electrons on the surface is thereby reduced or increased. This causes the liquid to be triggered to deliver or consume electrodes to compensate for this. The exchange of electrons per time, the electrode’s current, can be measured by the potentiostat. This is usually done in a three-electrode setup (Figure 2).
An electrode made to deliver current of a certain substance is present is called a sensor. This sensor has a certain sensitivity to the substance that you want to measure. For this reason, there are many different sensors that react in different ways to certain chemical substances.
Sometimes a sensor can be used multiple times, but often they are disposable, so for a one-time use, and the sensor is permanently changed by the reactions happening at the electrodes.