Hydrogen Peroxide
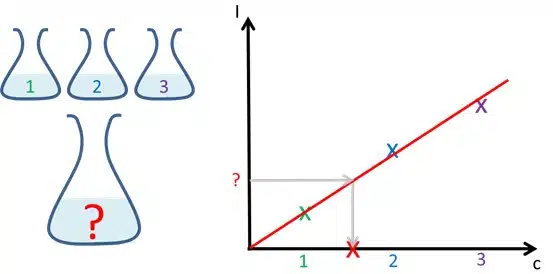
Detection of Hydrogen Peroxide 5/5 – The Calibration Curve
This chapter explains how to prepare and use a calibration curve.

Detection of Hydrogen Peroxide 4/5 – with Prussian Blue
This chapter is part of the series ‘Detection of hydrogen peroxide with Prussian Blue’. This chapter delves deeper into the specifics of detecting hydrogen peroxide using Prussian Blue.

Detection of Hydrogen Peroxide 3/5 – What is Prussian Blue?
Prussian Blue (PB) is a deep blue pigment that is hardly soluble in water, but little amounts that are dissolved or dispersed in watercolor the solution intensively blue. Small Prussian Blue particles dispersed in water have been used as the first modern synthetic color. It is also known as Berlin Blue or Turnbull’s Blue. The pigment is used for various paints (Paris Blue) or to make the blue color for blueprints.

Detection of Hydrogen Peroxide 2/5 – Why detect Hydrogen Peroxide?
In this chapter, we explain what hydrogen peroxide is and why we detect it. Hydrogen peroxide is the simplest peroxide (a compound with an oxygen-oxygen single bond). It is also a strong oxidizer. Due to its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent.
Detection of Hydrogen Peroxide 1/5 – Introduction
This series of articles aims to provide an introduction to electrochemical experiments. This series in particular deals with the detection of hydrogen peroxide with selfmade Prussian Blue electrodes, and includes an experiment (PDF) you can carry out yourself.
