Detection of Multiple Heavy Metals 4/5 – Stripping Analysis
This chapter is part of the series ‘Detection of Multiple Heavy Metals by Stripping Voltammetry’. In this chapter the theory on the analysis used in the corresponding experiment, namely stripping analysis, is explained.
The Stripping analysis
Heavy metals have an impact on the environment even in trace amounts; therefore methods to quantify them in trace amounts are needed. Currently, the stripping analysis is the most sensitive method that does not involve biomolecules for quantification. The stripping analysis adds a preconcentration step to the analysis. Imagine that the electrode first gets dressed and then strips.
This method is particularly suitable for heavy metals. In the preconcentration step, the electrode has a potential that is cathodic enough to reduce all the metals interesting for this analysis. The metal ions close to the electrode get reduced to the solid metal and deposit on the electrode. Multiple metals can be co-deposited that way.
One condition is that the metal sticks well to the electrode, otherwise the metal will detach from the electrode and there is no preconcentration on the electrode. Mercury forms alloys with most metals and is therefore suitable for stripping analysis, but working with mercury implies potential hazards. Mercury forms poisonous vapors and toxic organic compounds. Most labs try to avoid mercury.
For some metals certain other metal electrodes have the same effect, for example gold electrodes form alloys with deposited mercury, lead and other metals. Bismuth electrodes do this as well. However, mercury is still the golden standard for stripping analysis and new methods will always be compared to mercury electrodes.
The electrodes that will be used during this experiment, ItalSens IS-HM, are graphite electrodes modified with a mercury salt-containing polymer. They will be activated before the measurement and form a very thin mercury film on the electrode. This way the amount of mercury used for each analysis is minimized.
Step-wise method
The basic steps for a stripping analysis are:
The electrode is stabilized, cleaned, or modified in a way that is suitable for the application. For the ItalSens IS-HM this step will remove the metals from the last measurement.
A potential is applied to the electrode that leads to the reduction of the analyte. The solution is usually stirred during this step to increase the flux of metal ions to the electrode. Deposition times between 30 and 300 s are common for determining metal traces.
The stirring stops and the starting potential of the stripping step is applied. While in most cases this step allows the capacitive current to decay before the measurements start, during a stripping analysis this step allows the stirred solution to settle. A typical value is 30 s.
A voltammetric technique is applied to strip off the deposited species. Common methods are linear sweep voltammetry, differential pulse voltammetry, or square wave voltammetry. The potential will be swept to more anodic values and as a consequence the metals will be oxidized, desorbed, and dissolved.
The recorded data will be processed to calculate the amount of analyte in the sample.
Notes on the stripping analysis
The stripping analysis described here is known as anodic stripping voltammetry or analysis. The potential is swept to anodic (more positive or more oxidizing potentials) and the analytes are oxidized. These do not have to be metals; thiols adsorbed on gold or other combinations are possible as well. Another variant is the cathodic stripping voltammetry. Species are deposited at the surface due to oxidation and the potential sweep is towards more cathodic potentials (more negative). Examples of these processes are halides. They will form an insoluble salt with mercury ions on a mercury electrode. A cathodic sweep is reducing the mercury and releasing the halides again.
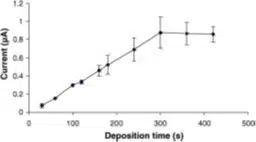
The preconcentration of the stripping technique and the sensitivity of SWV allows a quantification of concentration down to 10-10 M and 10-11 M. Furthermore a wider range of concentration with this method is accessible due to the possibility of tuning the deposition time.
The lower concentration limit of stripping voltammetry is reached if not enough species were adsorbed during the deposition step to create a significant current during the stripping. The upper limit is reached if multilayers of adsorbed species are formed during the deposition and cannot be completely stripped off. The formation of new compounds between adsorbed species can also limit the range of concentration that delivers a linear relationship with the current. Between these limits, an SWV should deliver a linear relationship between peak current and concentration of the corresponding species.
If a certain deposition time leads to a signal outside the linear range, the deposition time can be reduced to shift the linear range. A linear range is important to perform a standard addition analysis. If a new method is developed or if an analysis protocol is established, the limits of certain deposition times are evaluated and a suitable time for the expected concentrations is chosen (see Figure 3.1).
Literature
[1] S. Laschi, I. Palchetti, M. Mascini, Sensor Actuat B-Chem 114 (2006), p. 460 – 465
