Butler-Volmer equation
In general the Butler -Volmer equation describes the kinetics of a redox reaction close to the formal potential.
Applied to corrosion research the Butler-Volmer equation creates a relation between applied potential E, the measured current I, the corrosion potential Ecorr, corrosion current Icorr and the Tafel slopes for the anodic and cathodic reaction ßanodic and ßcathodic, also known as Tafel constants.
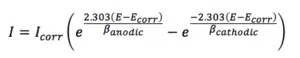
The Butler-Volmer equation is used in corrosion research as a model for a fit to a polarization curve. This way the corrosion current and corrosion potential can be extracted.
A modified version of the Butler-Volmer equation is the Stern-Geary equation.
Articles
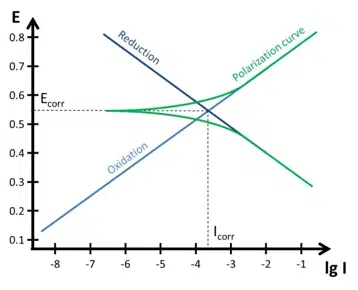
Tafel Plot and Evans Diagram
To understand the foundation of corrosion current measurements the Tafel plot and the Evan’s diagram are explained. The connection between a polarization curve and the Evan’s diagram is explained and how to extract the corrosion current from a polarization curve.
