Equivalent circuit
Electrochemical Impedance Spectroscopy (EIS) is complex and analysing the data is a demanding task. Using equivalent circuits and a fitting algorithm is a common way to make the analysis easier, more understandable, and visual.
An equivalent circuit is an circuit that is equivalent to the system in the electrochemical cell. Each effect or contribution to the total impedance is replaced by an electrical component. For example, a metal electrode in contact with an electrolyte containing a redox active species has 4 effects contributing to the impedance.
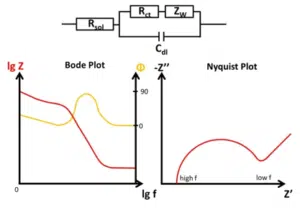
Warburg element in a schematic Bode and Nyquist plot
The current has to go through the solution between the working and counter electrode, which acts like an ohmic resistor, the solution resistance.
The current can flow in two ways through the electrode. One is an electrochemical reaction, i.e. transferring an electrode from the sulotion to the electrode or the other way around. The other way is charging up the electrochemical double layer, which acts like a capacitor.
The last contribution is the diffusion limitation or better the depletion of the active species in front of the electrode. The consequences of depletion cannot be simulated by classic electronic components. A new fictional element was created, the Warburg impedance.
The resulting circuit is known as the Randles Circuit.
The equivalent circuits for other systems, especially during corrosion research, are often more complex. However, by fitting the theoretical values of an equivalent circuit to the real measurement data, the contribution of the different components to the whole cell can be calculated.
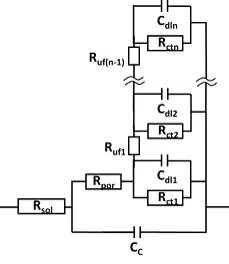
More information about Equivalent Circuits and fitting can be found in the corresponding article in the knowledge base.
Articles
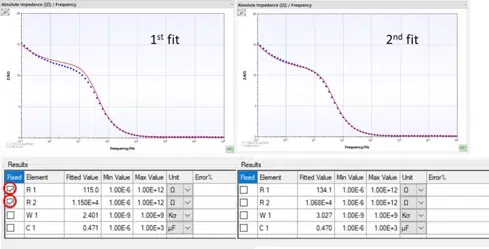
How to fit easily
In this section some guide lines to find good starting values for fitting are given.
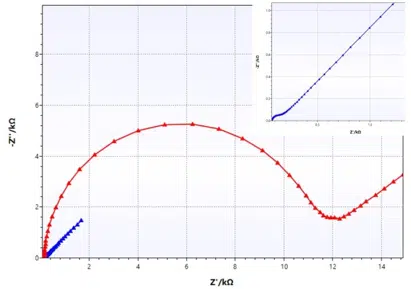
Equivalent circuit fitting for corrosion measurements
In this section of the handbook the Warburg Impedance and the Constant Phase Element (CPE) are introduced, which represent electrochemical effects with no corresponding real electronic components. Furthermore, some equivalent circuits for typical corrosion systems are presented.
