Working electrode
The working electrode is the place where the reaction happens that we want to control or investigate. As a consequence, this electrode should be carefully and reproducibly prepared. The most common working electrodes are disc electrodes.
For example, a metal cylinder or a metal wire is surrounded by Teflon or PEEK and the cross-section is exposed. The metal disc is connected to a wire at the other end of the coating, so it can be connected (see Figure 3.2). Common materials in electrochemistry for working electrodes are platinum and gold as well as a variety of carbon phases. Very popular due to its conductivity and reusability is glassy carbon.
In corrosion research, the working electrode is usually the surface or material that is to be studied. So in corrosion research most cells or sample holders focus on exposing a defined area to the solution in which the measurement is performed as well as providing an electrical connection of the sample (see Figure 3.3).
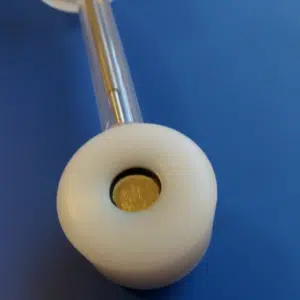
Another option to set up your sample is the Stern-Makrides arrangement. The sample has a cylinder shape and a hole with a screw thread. A Teflon cone is used to make the connection to the metal rod watertight (Figure 3.4). There are many other cells that allow insulating different parts of samples. There are cells for metal sheets or cells that are similar to a scanning droplet cell, where an electrolyte-filled tip is pressed to the surface and turns thus the part in contact with the liquid into a working electrode.
Rough surfaces will lead to high currents due to the charging of the interface, which will be explained in the chapter Capacitive Current. Dirty surfaces can show artifacts in the measurements or can be the cause that not the full active surface has access to the electrolyte. So cleaning an electrode is an important step in electrode preparation. A clean surface also increases the reproducibility of your measurements. It is therefore recommended to wear disposable gloves when assembling the samples and the holder.

The working electrode is the electrode where the investigated processes occur. It needs to be carefully prepared, so that the surface is reproducible and known.
Articles

Electrodes used with a potentiostat
This section gives a short overview of the three types of electrodes (working, reference, counter electrode) you will encounter while using a potentiostat. It is explained how these electrodes look like and what their task is.
